The Simple Physics Slogan
Hey you dummy, don't you know climate change is the simplest thing ever? It's so simple a child could explain it with a crayon. That's why it's settled. It's simple you see. So simple it's settled and there's no debate or doubt or uncertainty. Really. Well, except for the core concepts. And the measurement problems. And the modeling problems. And the discrepancies between models and reality. And all the stuff that we just don't know, which happens to be a lot of important stuff. What happens when we talk to a real expert and look at textbooks rather than comic books? Press play to find out, then press share and help get the discussion going.
TRANSCRIPT
Narrator
One of the most popular slogans in the climate debate says that the underlying science is not only settled, it’s simple.
John
I’m John Robson for the Climate Discussion Nexus, and this is a Fact Check video on the Simple Physics slogan.
Narrator
Al Gore called it “high school physics.”
NASA shows cartoons of a greenhouse to explain how the process works.
The National Geographic Magazine explains it this way: "The more greenhouse gases concentrate in the atmosphere, the more heat gets locked up in the molecules."
And kids go on TV to explain it to Donald Trump.
John
Gosh. It looks so simple even a Twitter Troll could understand it. Greenhouse gases warm the atmosphere like the walls of a greenhouse, and the heat gets locked up into the molecules.
Wait, how does heat get “locked” in molecules?
And if it’s high school physics, why are climate models so complex? And why does it take thousands of scientists to study the subject?
The answer is, it’s not simple. That’s just another empty slogan.
Narrator
At this point, in a traditional video about global warming, we’d start with our own cartoons. We’d show sunlight coming down and warming up the Earth, and then we’d show the heat going up into the air and a bunch of it getting back out through the blanket of greenhouse gases. And we’d show you cute pictures of snowmen waving or happy polar bears or something.
But we’re going to do something very different.
John
Instead of drawing a cartoon, I’m going to ask a physicist to explain what greenhouse gases do. And not to dumb it down. I’m going to ask what is actually going on at the molecular level, and how that affects the air temperature in the atmosphere. And I’m going to insist he use the proper scientific terminology, even if it means it’s hard to understand.
Let’s see what happens when we look at textbooks not comic books.
Narrator
William van Wijngaarden is a professor of physics at York University in Canada. He’s an expert on all things to do with temperature. He’s made detailed studies of the physics of energy at the molecular level. He’s shown how to cool down atoms to near absolute zero using lasers. And he’s also an expert on the climate system at the macro level, having published many studies of temperature and precipitation changes around the world.
William van Wijngaarden
The Basic Mechanisms
So the first question we want to ask is suppose there was no CO2, no greenhouse gases in the Earth’s atmosphere. What would happen?
Well, you would have the surface that would absorb sunlight and that would radiate heat to space. And the heat that is emitted from the surface would just go unimpeded to space, so basically there’s no blanket. So then it would be a lot colder than it is with greenhouse gases.
Now if you have an atmosphere with gases like CO2 and it isn’t just CO2 but water vapour, that’s the big greenhouse gas that people should talk about more, it’s water vapour, CO2, ozone, N2O and methane, those are the five big naturally occurring greenhouse gases.
If you have, say, a photon, or some heat, it goes up, it gets absorbed by one of those molecules. Well if that molecule has absorbed that heat, it will re-radiate it, but it will re-radiate in general in any direction. So some of the heat will come back down and some will come back up.
So it’s a bit difficult for that infrared radiation to just go out into space. Its trip to space is going to be slowed down and in being slowed down it turns out that results in the heating of the Earth’s surface.
Computing the Radiative Forcing of Greenhouse Gas Molecules
First consider: We have to ask ourselves what are the transitions where you can absorb this infrared light or heat. And by considering these five molecules, water vapour or H2O, CO2, ozone, N2O and methane, we’ve considered several hundred thousand different transitions.
So that’s one, just getting that data. Now there are libraries available where people have measured the frequencies and how strongly each of those frequencies is absorbed. So that’s Step 1.
Step 2 you have to know what’s the concentration of each of those molecules with altitude. So there you also rely on observations.
Step 3 is we need to know what’s the temperature versus altitude. So there are these standard temperature profiles that are also based on observation.
And then you have to calculate for each of those frequencies how each of those say 200,000 transitions absorb your different frequencies of light and you have to do that at every altitude. So that’s pretty involved.
The Logarithmic Relation Between CO2and Temperature
If you look at the greenhouse effect, the amount of warming, that depends logarithmically on CO2 concentration. So if I increase the CO2 concentration from 400 to 800 parts per million and get, say, a temperature increase of, say, 1 degree C, to get an additional 1 degree C of warming on top of that I can’t go from 800 to 1200, I have to go from 800 to 1600 parts per million. So it goes logarithmically. And that’s accepted by everyone.
Estimating the Temperature Effect of Doubling CO2 and Other GHGs
Well, what we’ve done in our work is we just consider those five gases. We see what happens if we double CO2, double methane, double N2O and have about a 6% increase in water vapour which corresponds to about a 1 degree C increase.
We find then that the temperature increase due to all those doublings is about 1 to 1.5 degrees C. That’s far below what many of those Armageddon folks like to talk about.
The big uncertainty is water vapour and especially clouds. And people don’t know what clouds do. If you have clouds during the day they block the sunlight and things cool down. If you have clouds at night they’re going to trap heat hence the temperatures stay warmer.
So are we going to have more clouds? Fewer clouds? We just don’t know.
Lots We Don’t Know
First of all, there are a lot of things we don’t know. We don’t know how to model ocean currents very well. Convection is extremely difficult to model, you’re dealing with a turbulent process, the equations are very complicated and no one can model that very well. So that’s why we have focused on radiation.
Right now we are unable to make a prediction of how turbulence, those fluid equations, how that behaves. That’s just too hard for us to model. And even if we get much, much faster computers that’s going to remain a very, very tough problem.
The H2O Continuum
Some basic physics that even isn’t very well understood: For example, people like to talk about when you calculate the absorption of these different wavelengths, they say OK you have all these different lines for CO2, H2O etc. So then they make some predictions based on those lines of absorption and then they look at observations and then they look for the difference and they find that there’s a big difference and that they say is due to something called the H2O continuum. Well you ask “What is the H2O continuum due to?” and no one seems to know.
So when you ask, is this well understood, no it’s not. I think the problem in this field is people have not said, make some predictions, what are the observations, is there agreement between the model and the observations? And sometimes these models just have failed abysmally.
That doesn’t mean the modelers are dumb folks. But it’s just a very difficult thing sometimes to model. Climate is not simple to model.
John
Oh dear. That sounded disquietingly like real science. The kind you might study at college and fail. And there's no doubt we lay people need to try to keep up to speed on that and all kinds of other scientific subjects, partly to be informed citizens on important policy issues and partly just to be well-rounded. But part of understanding science is understanding where the complexities lie, and not getting browbeaten, especially by people who aren't scientists or won't admit science is complex, into believing it's so simple a child can explain it with a crayon.
That’s not true of economics. It's not true of foreign policy. It's not true of how our system of government works, or all sorts of other things we want to understand in order to make informed decisions. And it's not true of climate science.
For the Climate Discussion Nexus, I'm John Robson. Thanks for watching our Fact Check video on the Simple Physics slogan, and we’ll see you next time.
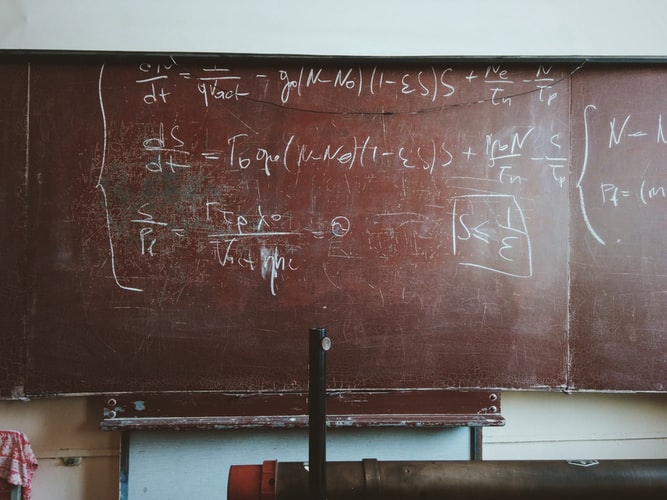


Greenhouses work by supressing convection, not IR emission. Carbon dioxide is a greenhouse gas because it is used in greenhouses to encourage tomatoes! So basically the term GHG needs quotation marks. Incidentally a very large number of our terms need quotations, eg. "Liberal", "Conservative", "Newspaper". You get the drift.
PS. John your commentaries are extremely good. Facts and wit. I have come to a very Bristish conclusion that much of what troubles us needs to be laughed at, loudly.
PPS. Christopher Monckton. He would love your stuff.
This simple mind still has some simple questions:
If there is global warming and ozone is a "geenhousegas", why did we have to "fight" to close the hole in the ozone layer?
Whasn't that just an exhaust valve for excessive heat? Maybe a natural feedback from mother earth?
Another question I have: How can geothermal technic be good for the climate, if it brings heat from the inner earth to the atmosphere?
Just simple questions with probably no simple answer.
Ozone in the stratosphere blocks ultraviolet light which is dangerous to plants and animals. Ozone in the troposphere acts as a greenhouse gas.
Please leeward out the music, it's very distracting. Otherwise very nice video.
Ozone is an unstable form of oxygen. It 'blocks' uv frequencies by absorbing the energy of the photons. It naturally decays back to O2 at a rate dependent on temperature. The ozone hole appears in the hemisphere that is in winter because the uv levels coming in are low.
I am not a scientific person but I do know that humans ,plants and animals couldn't live without co2,that is basically high school science ??
This is a very misleading video, which does not deliver a balanced and complete overview of the basic physical principles of climate change and climate science. The scientist being interviewed makes statements which are also quite misleading, such as those about our ability to model ocean currents - this guy should be ashamed of himself.
Ben, the was no discussion of ocean currents. He state that convection was hard to model. This is the transfer of heat between the ocean surface and the atmosphere, an interface of two turbulent fluids. This interface may be impossible to model.
Thanks for providing sources that alarmists have been using simple explanation to sum up the basic core mechanic of what the scientists have found out in their hard work. William van Wijngarden reconfirmed this core mechanic in his more sophisticated Explanation.
It would have been nice to also provide any sources whatsoever for what he then goes on to say about not knowing certain interactions. Yes, he is a scientist, but I'd still like to see scientific sources on a scientific claim like this.
But just like you did with the hockey stick video and Keith Briffa, you know not to provide easy-to-find sources when you know that they will prove you wrong. Classic CDN.
Well Ben, what didn't he say and why are you correct but he, a physicist of great repute, not?
Mariasabina - Do you have any scientific argument against what Wijngaarden says or do you just insist on making noise and waving your arms around?
I understand that the paper by Wijngaarden and Happer has been submitted to a journal. The fact that he hasn't published previous (if you are correct) doesn't mean anything.
I WILL TELL YOU, AS A FORMER GEOPHYSICIST: GLOBAL WARMING HAPPENS UNDER PURE PHYSICAL LAWS, AND NOT BECAUSE OF THE “QUOTA ON CARBON GAS”
By Alexander Gorodnitsky
More than two thousand years ago, astronomers of ancient Egypt discovered the phenomenon of precession (the axis of the Earth moves in a circle relative to the tilt to the Sun). Known to many since childhood, the toy "spinning top" (yule) before stopping begins to swing.
The same phenomenon occurs with the Earth and is called - precession.
The Earth in rotation around its axis slows down the movement (for about a thousand years for one second).
Moreover, with a fading rotation, it sways like a whirligig, substituting one or another place for the Sun.
The same as a person sitting by the fire turns to the fire, one side or the other. What is the meaning of the rays of the sun for the earth, everyone knows.
In precession, one complete revolution of the Earth’s axis in a circle (360 degrees) occurs in about 25765 years.
For 72 years, the movement of one degree. The angle of inclination of the axis of the Earth (from the conditional centre) when rocking is 23 degrees and 27 minutes.
What is 23 degrees latitude on Earth? This is how the distance from Sweden to Cyprus.
The angle between the maximum and minimum inclination of the Earth to the Sun in the precession is twice as large and is 46 degrees and 54 minutes.
And this is, approximately, as the distance from the Arctic Circle to Africa.
One climate will be when the Earth is substituted for the rays of the Sun by the coast of Africa and quite different when it turns into the coast of Scandinavia.
Thus, the entire climate on Earth (temperature, humidity, water level in the world's oceans, flora and fauna, etc.) does not depend on a conference in Paris, but on the phenomenon of the Earth’s precession (the inclination of the planet toward the Sun).
Geological knowledge says that the last ice age in Europe ended about 12,000 years ago.
The ice shell sometimes descended to the breadth of cities - Kyiv, Berlin, London.
Scandinavia, the Baltic, Canada and Siberia were under a meter-long layer of ice and snow. The ice was as it is today in Antarctica.
Because ice covered a large area of Eurasia and America - water was concentrated in it.
In addition, from this, the world ocean level was 150-200 meters lower than the modern one.
Now let us think ... If the peak of cooling was 12 thousand years ago, then with the precession movement of the axis of the Earth, the peak of heat in the Northern Hemisphere is still not passed.
Real heat (its peak) will be in a thousand years. Until then, there will be constant warming.
Now on Earth for the Northern Hemisphere, according to the years of the precession, it is approximate “the beginning of summer”.
After passing the peak of heat on Earth by “inertia", another four thousand years will be warming up.
The consequences can also be predicted.
Modern deserts will expand, steppes will dry up, forest zones will turn into steppes, and swamps in the tundra will dry out and become overgrown with deciduous forests. The Arctic Ocean will be moderately warm. Winters will be snowless.
In the next thousand years, all ice in the entire Northern Hemisphere will melt, and the sea level will rise from this.
With the melting of the northern ice under the sun, the melted water will heat up. Warm "northern water" by ocean currents will melt the ice of Antarctica and this will further raise the overall water level in the seas and oceans.
As a result, large areas of modern coasts and cities will be under water.
Nevertheless, today you should not worry about all this, since by that time there will be completely different values and priorities in the life of humankind.
No one today knows how high the temperature on the Earth will rise.
Of course, it is possible to simulate a situation, but humankind is not able to change the precession climate change.
The situation must be accepted, as well as the presence of the Sun in the sky.
After a thousand years, the Earth, having reached the peak of heat in the Northern Hemisphere, will go in its opposite direction in its precession movement.
Moreover, after 14 thousand years it will reach the “peak of the cold”. In the Northern Hemisphere, the ice age will again begin.
A thick (several hundred meters or more) layer of ice will cover Scandinavia and northern Europe, Siberia and Canada. Earth's water will concentrate in this ice; the level of the oceans will again fall by two hundred or more meters.
From the cold of the vast northern ices over the whole Earth, it will become very cold.
Antarctica during the "peak of cold" will be cleared of the ice and will be a liveable blooming green continent.
Therefore, on the Earth it will occur cyclically every 25,920 years.
Humanity is still young with knowledge. In fact, it is in “infancy” and lives its first conscious life in the history of the Earth. Because everything that happens on Earth - everything for the education of humankind, is happening for the first time.
Humanity can be a happy observer of the precession climate change on Earth with cyclical periods of warming and cooling.
However, he may not see anything of this if before, through his stupid self-interest, he would exterminate themselves in an internecine struggle, for example, to redistribute “carbon dioxide quotas” or in thermonuclear wars for money, power and the ambitions of leaders.
I saw this video several months ago and just seeing it again now was well worth it. William van Wijngarden is an impressive scientist. He clearly and simply portrayed the complexity of the science involved with H2O, CO2, N2O, ozone and methane and infrared radiation.
hello
"So the first question we want to ask is suppose there was no CO2, no greenhouse gases in the Earth’s atmosphere. What would happen?" And the first answer should be that without CO2, all life would be dead - that is 200 year old biology. The CO2 "fertilization effect" is an understatement. It is only appropriate for the range of increased CO2 used in greenhouses. (up to 1600ppm, which produces the strongest, healthiest, most beautiful and productive plants) Cyanobacteria took one or two billion years to create ANY free O2, let alone our current 20%. All of the first O2 had to oxidate (rust) all of earth's surface iron before any free O2 could form. ALL life's energy and all our atmosphere's oxygen comes through photosynthesis: sunlight plus CO2 + water gives sugar + O2. CO2 is FAR MORE IMPORTANT TO BIOLOGY THAN IT IS TO CLIMATE. The sugar produced by photosynthesis provides ALL OF THE ENERGY REQUIRED FOR LIFE ON EARTH - MAKING CO2 along with water THE BASIC INGREDIENTS FOR ALL LIFE ON EARTH. AND CO2 CONCENTRATIONS HAVE BEEN DECLINING FROM CONCENTRATIONS TEN TO TWENTY TIMES THAT OF TODAY'S AT THE BEGINNING OF MULTICELLULAR EVOLUTION NEARLY 600 MILLION YEARS AGO. DECLINING TO TODAY'S NEAR LETHAL LOWS (all life begins to die at CO2 levels of 100-150ppm). During glacial phases of our ongoing Pleistocene/Holocene ice age, CO2 concentrations drop to within 30ppm of lethal lows. That is because CO2 dissolves MORE AS TEMPERATURES DROP. And CO2 outgasses from water as temperatures rise increasing atmospheric CO2. The natural relationship is temperature CAUSING CO2 concentration changes (CO2 lags by about 800 years) - not the reverse. And making the 180-280ppm natural range of CO2 during our ongoing Pleistocene/Holocene ice age the COLDEST (and nearest to lethal) RANGE THAT EARTH HAS EVER ENDURED during multicellular evolution. Far too close to lethally LOW LEVELS OF CO2. While EVERY MEASURE OF CLIMATIC TEMPERATURE REMAINS WELL WITHIN NATURAL RANGES. Well within the one thousand year Eddy cycle's 4 degree C range which bottomed out in the 16th century and which will top out after warming another 2 degrees C naturally. Well within the natural variations of our current ongoing Holocene twelve thousand year interglacial. And well within our current ongoing Pleistocene/Holocene ongoing ice age - three million years so far - the coldest the earth has ever endured. And since the climate remains well within its natural temperature ranges of the last three million years, in spite of CO2 levels not seen in twelve million years. The 41% increase in CO2 since the mid-twentieth century has nothing but a Godsend to life on earth - and insignificant to climate.
Hi Rod, CO2 is used in green houses because higher concentrations of it will help plants grow faster. Studies have shown this. Its not about it being a ‘greenhouse gas’. Who came up with that term anyway as a green house gas implies heat retention and that is not why greenhouses use CO2.
Just an excellent overview you wrote Barry. You did not mention that the sun has variable heat radiation cycles as well. I thought sun cycles was the major cause of climate change, from ice ages to warm periods. The titling of the earths axis and it changes both cools the north and warms the south for half a year, then warms the north and cools the south another half of the year. Effectively cancelling the effects out over a year. Im not understsnding your explanation about the axis impacts over thousands of years.
To: Barry Bateman and Andy Oliver
Gentlemen:
Excellent write up by both of you and you are right on the money. I have to admit I'm late to the dance here. I have been looking at this issue for only the past 2 years. About 1 year ago, I came to the conclusion that what's missing in this whole debate is a good old fashion experiment of sufficient scale to prove, beyond the shadow of a doubt, that CO2 is or is not, a "heat retention" molecule at all. I have taken steps to do exactly that. It's called the ATMOSPHERIC CLIMATE TESTING SIMULATOR and you can find it on my website at http://www.dextras.com/climate.html . Look it up and let me know what you think. The web site has my email address shown on the contact link and so you can reach me through there. Look forward to hearing from you.
P.S. I have also since discovered that the alarmists hero, Arrhenius, was proposing exactly that in his 1896 paper. No one took him up on it and that's why we are in the mess we are in today. I've done a small write up on it and I can send it to you directly if you like.
MEMORANDUM
To: Climate Discussion Nexus Subscribers December 11, 2022
From: Kenneth G. Dextras, B. Eng., McGill ‘76
Subject : CDN video “ Debunking the ‘Simple Physics’ slogan“
Dear Fellow Subscribers:
I had another look at the above video and I’m sorry to say that Professor Wijngaarden and Al Gore are both missing the boat almost entirely. Gore’s simplistic CO2 greenhouse gas allegation is pure fantasy. It is and continues to be an unproven and religious like statement that yes, even a child would accept because fantasy is the world he lives in. Gore then raises the child to teenager level and then proclaims it as obvious as high school physics while completely ignoring the basic equations of state of high school physics namely: PV = nRT and
C = (dQ/dT)/m . Even a teenager would know that if the heat source in any thermodynamic system is constant and you don’t materially change n or m, you’re not going to change the temperature of any substance, solid, fluid or gas. Of course we all know why Gore ignores this “simple” physics because if he did not, his whole CO2 theory would vanish into thin air, pardon the pun.
Unfortunately, Wijngaarden makes matters worse by also ignoring these irrefutable facts and instead, wanders off into the abyss of “it’s complicated” (convection, clouds etc…) and thus bogs himself down into the minutiae of “data”. What he seems to have completely forgotten is that the atmosphere as a whole is nothing more than a special case of the 1st Law known as the free expansion; the fundamental axiom of which is: THERE IS NO WORK DONE ON THE SYSTEM AS A WHOLE IN A FREE EXPANSION.
Understanding this fundamental principle simplifies matters considerably. First, it eliminates the time lag to (P/V) equilibrium entirely such that the response time to any outside influence such as heat is, in effect, INSTANTANEOUS. Secondly, duplicating Joules experiments of the free expansion of air but using more modern measuring equipment, showed a small DECREASE in temperature. This means that small increases in the heat input are essentially cancelled by the expansion. It’s like having a built in fly-ball governor regulating the temperature of the atmosphere and so, it’s not surprising that actual solar input and air temperature measurements in the troposphere during the past 60 years confirms these principles almost exactly. It could not be otherwise because as Einstein once said of thermodynamics: “ It is the only physical theory of universal content which I am convinced, within the areas of the applicability of its basic concepts, will never be overthrown.”
In effect, what the above shows is that the atmosphere is generally a very stable thermodynamic system with one sole exception – temperatures near the surface. Again, this makes perfect sense since the earth itself is an extremely non-uniform mixture of rocks, soil, water and plants of infinite variety so the internal mixing of air above it (diurnal wind effect) will always be volatile and yes, even violent at times. This begs the obvious question: so what ? Do you really think you can work backwards from this “chaos”, that varies everywhere every day, and come up with some kind of math model that can realistically and reliably predict the future ? No you can’t and the fact that the algorithmic models of today are way off the mark proves it beyond the shadow of a doubt so why go there anymore ? It doesn’t work because it can never work. It’s nothing more than a perpetual grant machine for academia that has a vested interest in making it a lot more complicated than it really needs to be. It’s time for all of us to get off that train and here’s how you do it. Start actively spreading the word about the ACTS (see http://www.dextras.com/climate.html ) as the only practical option for putting an end to this irrational childish debate regarding CO2. Remember: “It is a mark of an educated man and a proof of his culture that in every subject, he looks for only so much precision as its nature permits.” (Aristotle, 350 bc) and: ” Hypotheses cannot be freely invented but must have some experimentally verifiable aspect.” (Michael Faraday, 1860). If you agree or disagree on any of this, let me know. My email is on the web site. Look forward to hearing from you.